Bulbs
Flower Basics
Flower Beds & Specialty Gardens
Flower Garden
Garden Furniture
Garden Gnomes
Garden Seeds
Garden Sheds
Garden Statues
Garden Tools & Supplies
Gardening Basics
Green & Organic
Groundcovers & Vines
Growing Annuals
Growing Basil
Growing Beans
Growing Berries
Growing Blueberries
Growing Cactus
Growing Corn
Growing Cotton
Growing Edibles
Growing Flowers
Growing Garlic
Growing Grapes
Growing Grass
Growing Herbs
Growing Jasmine
Growing Mint
Growing Mushrooms
Orchids
Growing Peanuts
Growing Perennials
Growing Plants
Growing Rosemary
Growing Roses
Growing Strawberries
Growing Sunflowers
Growing Thyme
Growing Tomatoes
Growing Tulips
Growing Vegetables
Herb Basics
Herb Garden
Indoor Growing
Landscaping Basics
Landscaping Patios
Landscaping Plants
Landscaping Shrubs
Landscaping Trees
Landscaping Walks & Pathways
Lawn Basics
Lawn Maintenance
Lawn Mowers
Lawn Ornaments
Lawn Planting
Lawn Tools
Outdoor Growing
Overall Landscape Planning
Pests, Weeds & Problems
Plant Basics
Rock Garden
Rose Garden
Shrubs
Soil
Specialty Gardens
Trees
Vegetable Garden
Yard Maintenance
What Is Alkaline pH?
What Is Alkaline pH?. With respect to the chemical concept of pH, "alkaline" is another term for "basic." These two terms are interchangeable. The pH scale was developed to give a numerical value that corresponds to how acidic or basic a material is. Acids have a pH from 0-7, while bases have a pH from 7-14. A pH of exactly 7.0 is neutral,...
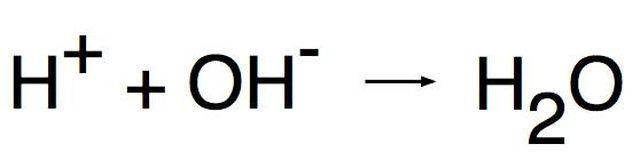
With respect to the chemical concept of pH, "alkaline" is another term for "basic." These two terms are interchangeable. The pH scale was developed to give a numerical value that corresponds to how acidic or basic a material is. Acids have a pH from 0-7, while bases have a pH from 7-14. A pH of exactly 7.0 is neutral, indicating that a substance is neither acidic nor basic. Although plants tolerate a small range of soil pH quite well, extremely acidic or alkaline soils need to be adjusted for good plant health.
Acids and Bases
The easiest to understand definition of an acid or base is the Arrhenius definition (named after the Swedish chemist Svante Arrhenius). According to this definition, an acid is a substance that generates hydronium ions when dissolved in water. A hydronium ion is a hydrogen nucleus without its usual single orbiting electron. These ions have a positive electrical charge and the chemical symbol H+.
A base generates hydroxide ions when dissolved in water. Hydroxide ions have a negative charge and the symbol OH--. Hydroxide ions are left with one more electron than proton, giving rise to the negative charge.
A substance is acidic if it contains more H+ than OH- ions, and it is alkaline if the OH- concentration is greater. Hydrogen chloride (HCl) becomes hydrochloric acid when dissolved in water to form H+ and Cl- ions. Sodium hydroxide (NaOH) is a base because in solution it dissolves to form Na+ and OH- ions.
The pH Scale
The pH scale was developed to provide an easy numerical description of acidity and alkalinity. The scale ranges from 0 to 14 with the number in the middle (7) representing a point where the number of hydroxide ions and hydronium ions is equal. Substances with a pH of 7 are said to be "neutral."
The pH scale is logarithmic, meaning that the difference in acidity or alkalinity from one whole pH value to the next changes by a factor of ten. The concentration of hydronium ions at pH 4 is ten times greater than it is at pH 5. Likewise, a pH of 9 represents a concentration of ten times as many hydroxide ions compared to pH 8.
The pH Preferences of Plants
With some exceptions, most plants do well in a slightly acidic to neutral soil (pH 5.5-7). It is unlikely there will be a noticeable difference in the health of a plant grown at pH 6.5 compared to one grown at 6.7. There is no need to expend a lot of effort in closely regulating pH.
Some common garden plants and their tolerable pH ranges are: tomato (5.5-7.5); lettuce (6-7); cucumber (5.5-7); blueberry (4.5-5.5) and rose (5.5-7).
Always find out the acceptable pH range for the plants being grown, and adjust the pH to within a few tenths of the middle of the range. Test the soil periodically, because the pH of many soils will tend to drift back to the original "natural" value.
Alkaline Soils
Alkaline soils are more often encountered and are more problematic than acidic ones. Soils over limestone bedrock tend to have a high pH (7-9), and few plants will do well in them. At pH above 7, phosphorus becomes unavailable, and the build up of calcium carbonate and other toxic salts can inhibit root development. Soil pH varies widely in any given region, so soil should be tested before planting.
Soil pH can best be lowered to an acceptable range in one of two ways. Depending on initial and desired conditions, elemental sulfur (NOT sulfate) can be added at a rate of 1-8 pounds per one hundred square feet. This method works well but takes time to have its affect. The job can be done faster by adding the acid-forming salts ammonium sulfate or ammonium nitrate. A strong ammonia smell may emanate from the soil during this time, but that is normal.
Testing Soil pH
Low cost soil test kits are available from most garden centers or mail order gardening suppliers. They are not highly accurate but are sufficient for the needs of a gardener.
A more accurate and detailed analysis can obtained by sending a soil sample to a testing laboratory. Commercial services will charge from $10-$30, depending on the amount of information offered. State agricultural extension services often provide soil testing services for a nominal fee or no charge.